My long-term interest is to study endogenous properties of single inhibitory interneuron that allows them to serve as information capture and storage devices in the brain. Towards this end, I perform two-photon Ca2+ imaging, electrophysiological recordings and optogenetic manipulations from in vivo cortical circuits, combined with molecular perturbations including CRISPR-Cas9 mediated genome editing, to identify cellular mechanisms within or between neurons that enable their retention and consolidation of information, integration of sensory inputs, coincidence detection and refinement of topographic sensory maps.
Currently, as a Marie Curie Fellow and together with a NARSAD grant, I continue studying neural circuit deficits in Autism Spectrum Disorders, reward processing and goal-directed learning in awake behaving animals, essentially building a bridge between mesoscopic neural circuits encoding specific behavior.
Interneurons and Cortical Plasticity
Interneuron deficits in Neuropsychiatric Disorders
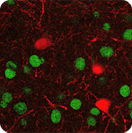
Rett syndrome (RTT) arises from loss of function mutations in Mecp2 in the brain, but fundamental aspects of its physiological mechanisms are unresolved. Here, by whole-cell recording of synaptic responses in visual cortex of MeCP2 mutant mice in vivo, we show that visually-driven excitatory and inhibitory conductances are both reduced in cortical pyramidal neurons. The ratio of excitation-to-inhibition (E/I ratio) is increased in amplitude and prolonged in time-course. These changes predict circuit-wide reductions in response reliability and selectivity of pyramidal neurons to visual stimuli, as confirmed by two-photon imaging from MeCP2 mutant mice. Targeted recordings reveal that parvalbumin-expressing (PV+) inhibitory interneurons in mutant mice have reduced responses. PV-specific MeCP2 deletion alone substantially recapitulates effects of global MeCP2 deletion on cortical circuits, including reduced pyramidal neuron responses and reduced response reliability and selectivity. Furthermore, MeCP2 mutant mice show reduced expression of the Cl- cotransporter KCC2 and reduced KCC2/NKCC1 ratio. Perforated patch recordings demonstrate that the reversal potential for GABA is more depolarized in mutant mice, but is restored by application of the NKCC1 inhibitor bumetanide. Treatment with recombinant human IGF1 restores responses of PV+ and pyramidal neurons, including their reliability and selectivity, and increases KCC2 expression to normalize the KCC2/NKCC1 ratio. Thus, loss of MeCP2 in the brain alters both excitation and inhibition in brain circuits via multiple mechanisms. Loss of MeCP2 from a specific interneuron subtype contributes crucially to the cell-specific and circuit-wide physiological deficits of RTT. The joint restoration of inhibition and excitation in cortical circuits is pivotal for functionally correcting the disorder.
Genome Engineering in Neuropsychiatric Disorders